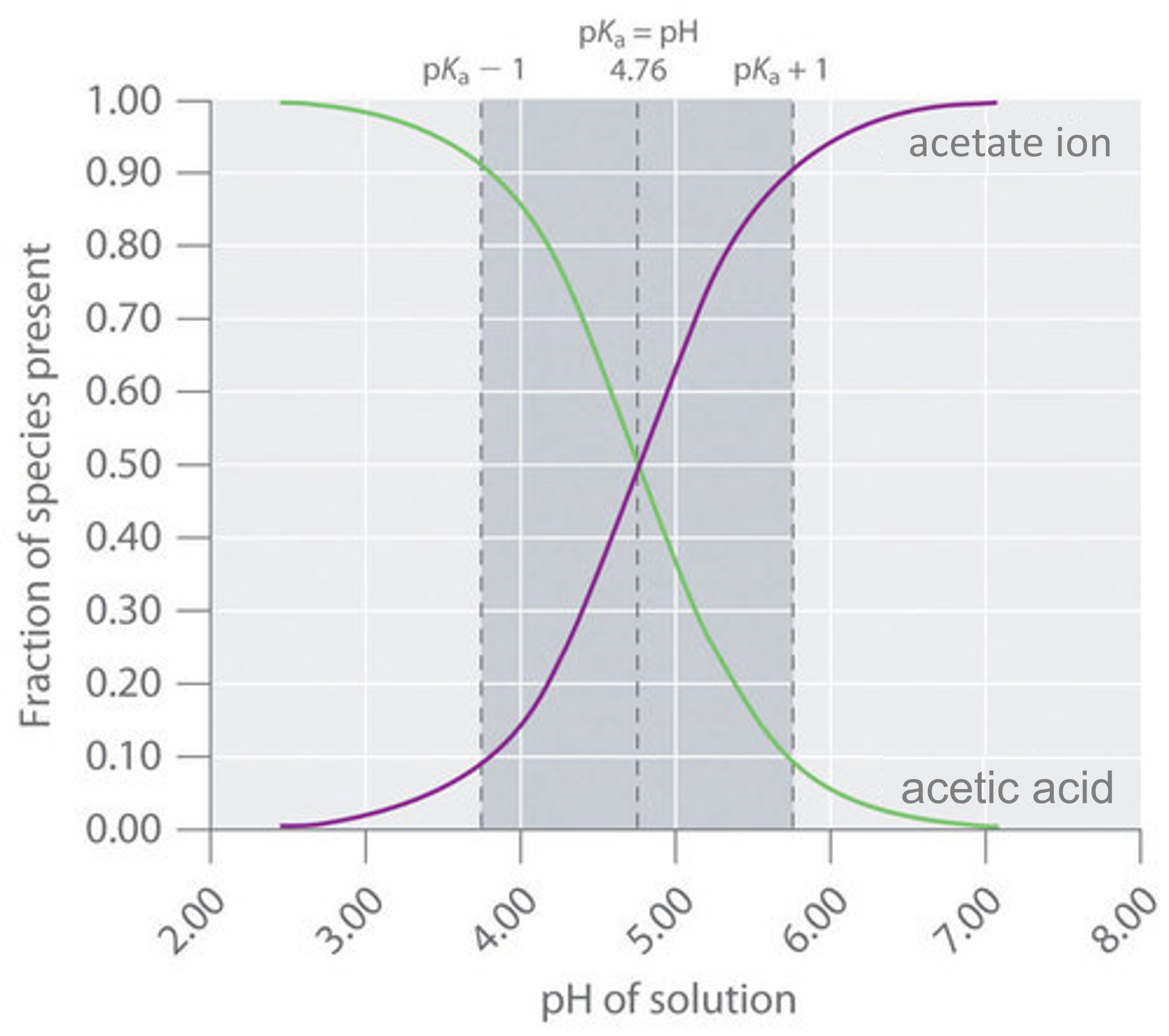
Dilution Of Buffer Solution Effect On Ph . Diluting a buffer solution would decrease its buffer capacity. It can be also defined as the quantity of strong acid or base that must be added to change the ph of one liter of solution by one ph unit. Let us take a look on the following example in the. The buffer capacity considering the effect of dilution, β dil, is defined as the added amount of strong base or strong acid required to change in one unit the ph of an initial v o. The effects of dilution on the ph of a strong acid (unbuffered solution) and a weak acidic buffer are shown in the diagram below. The most important of these is undoubtedly the h 2. If you look at the buffer formula, ph = pka + lg [salt]/[acid], dilution does. Generally, dilution has no effect on the ph.
from chem.libretexts.org
It can be also defined as the quantity of strong acid or base that must be added to change the ph of one liter of solution by one ph unit. If you look at the buffer formula, ph = pka + lg [salt]/[acid], dilution does. Generally, dilution has no effect on the ph. Let us take a look on the following example in the. The most important of these is undoubtedly the h 2. The effects of dilution on the ph of a strong acid (unbuffered solution) and a weak acidic buffer are shown in the diagram below. The buffer capacity considering the effect of dilution, β dil, is defined as the added amount of strong base or strong acid required to change in one unit the ph of an initial v o. Diluting a buffer solution would decrease its buffer capacity.
8.8 Buffers Solutions That Resist pH Change Chemistry LibreTexts
Dilution Of Buffer Solution Effect On Ph Generally, dilution has no effect on the ph. It can be also defined as the quantity of strong acid or base that must be added to change the ph of one liter of solution by one ph unit. The most important of these is undoubtedly the h 2. Diluting a buffer solution would decrease its buffer capacity. The effects of dilution on the ph of a strong acid (unbuffered solution) and a weak acidic buffer are shown in the diagram below. If you look at the buffer formula, ph = pka + lg [salt]/[acid], dilution does. Let us take a look on the following example in the. The buffer capacity considering the effect of dilution, β dil, is defined as the added amount of strong base or strong acid required to change in one unit the ph of an initial v o. Generally, dilution has no effect on the ph.
From www.studypool.com
SOLUTION Ph pka pkb buffer solution Studypool Dilution Of Buffer Solution Effect On Ph The effects of dilution on the ph of a strong acid (unbuffered solution) and a weak acidic buffer are shown in the diagram below. If you look at the buffer formula, ph = pka + lg [salt]/[acid], dilution does. The buffer capacity considering the effect of dilution, β dil, is defined as the added amount of strong base or strong. Dilution Of Buffer Solution Effect On Ph.
From www.youtube.com
Calculating the pH of a buffer YouTube Dilution Of Buffer Solution Effect On Ph The most important of these is undoubtedly the h 2. If you look at the buffer formula, ph = pka + lg [salt]/[acid], dilution does. The effects of dilution on the ph of a strong acid (unbuffered solution) and a weak acidic buffer are shown in the diagram below. Let us take a look on the following example in the.. Dilution Of Buffer Solution Effect On Ph.
From www.slideserve.com
PPT Buffer This PowerPoint Presentation, free download ID2841454 Dilution Of Buffer Solution Effect On Ph Generally, dilution has no effect on the ph. It can be also defined as the quantity of strong acid or base that must be added to change the ph of one liter of solution by one ph unit. Diluting a buffer solution would decrease its buffer capacity. The buffer capacity considering the effect of dilution, β dil, is defined as. Dilution Of Buffer Solution Effect On Ph.
From chemistry.stackexchange.com
acid base Calculation of pH in diluted buffer. Chemistry Stack Exchange Dilution Of Buffer Solution Effect On Ph Generally, dilution has no effect on the ph. Diluting a buffer solution would decrease its buffer capacity. The effects of dilution on the ph of a strong acid (unbuffered solution) and a weak acidic buffer are shown in the diagram below. Let us take a look on the following example in the. The buffer capacity considering the effect of dilution,. Dilution Of Buffer Solution Effect On Ph.
From www.researchgate.net
pH change in different concentrations of phosphate buffer with 5 mM Dilution Of Buffer Solution Effect On Ph The most important of these is undoubtedly the h 2. If you look at the buffer formula, ph = pka + lg [salt]/[acid], dilution does. Generally, dilution has no effect on the ph. Diluting a buffer solution would decrease its buffer capacity. It can be also defined as the quantity of strong acid or base that must be added to. Dilution Of Buffer Solution Effect On Ph.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Dilution Of Buffer Solution Effect On Ph Generally, dilution has no effect on the ph. Let us take a look on the following example in the. The most important of these is undoubtedly the h 2. Diluting a buffer solution would decrease its buffer capacity. The buffer capacity considering the effect of dilution, β dil, is defined as the added amount of strong base or strong acid. Dilution Of Buffer Solution Effect On Ph.
From chem.libretexts.org
8.8 Buffers Solutions That Resist pH Change Chemistry LibreTexts Dilution Of Buffer Solution Effect On Ph If you look at the buffer formula, ph = pka + lg [salt]/[acid], dilution does. The buffer capacity considering the effect of dilution, β dil, is defined as the added amount of strong base or strong acid required to change in one unit the ph of an initial v o. The most important of these is undoubtedly the h 2.. Dilution Of Buffer Solution Effect On Ph.
From www.youtube.com
Calculating pH of buffer solution with Strong Acid YouTube Dilution Of Buffer Solution Effect On Ph Diluting a buffer solution would decrease its buffer capacity. The effects of dilution on the ph of a strong acid (unbuffered solution) and a weak acidic buffer are shown in the diagram below. The buffer capacity considering the effect of dilution, β dil, is defined as the added amount of strong base or strong acid required to change in one. Dilution Of Buffer Solution Effect On Ph.
From www.slideserve.com
PPT Hydrolysis of Salts and pH of Buffer Solutions PowerPoint Dilution Of Buffer Solution Effect On Ph Generally, dilution has no effect on the ph. The buffer capacity considering the effect of dilution, β dil, is defined as the added amount of strong base or strong acid required to change in one unit the ph of an initial v o. The most important of these is undoubtedly the h 2. Let us take a look on the. Dilution Of Buffer Solution Effect On Ph.
From cobasaigonjp.com
How To Prepare A Buffer Solution Preparing Your Own Buffer Solutions Dilution Of Buffer Solution Effect On Ph The effects of dilution on the ph of a strong acid (unbuffered solution) and a weak acidic buffer are shown in the diagram below. Let us take a look on the following example in the. It can be also defined as the quantity of strong acid or base that must be added to change the ph of one liter of. Dilution Of Buffer Solution Effect On Ph.
From labpedia.net
Solutions Part 3 Solutions with various examples Dilution Of Buffer Solution Effect On Ph Diluting a buffer solution would decrease its buffer capacity. If you look at the buffer formula, ph = pka + lg [salt]/[acid], dilution does. The buffer capacity considering the effect of dilution, β dil, is defined as the added amount of strong base or strong acid required to change in one unit the ph of an initial v o. The. Dilution Of Buffer Solution Effect On Ph.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Dilution Of Buffer Solution Effect On Ph Diluting a buffer solution would decrease its buffer capacity. Generally, dilution has no effect on the ph. The effects of dilution on the ph of a strong acid (unbuffered solution) and a weak acidic buffer are shown in the diagram below. The buffer capacity considering the effect of dilution, β dil, is defined as the added amount of strong base. Dilution Of Buffer Solution Effect On Ph.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Of Buffer Solution Effect On Ph It can be also defined as the quantity of strong acid or base that must be added to change the ph of one liter of solution by one ph unit. Let us take a look on the following example in the. The most important of these is undoubtedly the h 2. The effects of dilution on the ph of a. Dilution Of Buffer Solution Effect On Ph.
From iu.pressbooks.pub
Buffers, Indicators, and Solution Standardization IU East Dilution Of Buffer Solution Effect On Ph If you look at the buffer formula, ph = pka + lg [salt]/[acid], dilution does. Let us take a look on the following example in the. The effects of dilution on the ph of a strong acid (unbuffered solution) and a weak acidic buffer are shown in the diagram below. The most important of these is undoubtedly the h 2.. Dilution Of Buffer Solution Effect On Ph.
From www.researchgate.net
Effect of solution pH, buffers, NaCl, and arginine on the PEG curves Dilution Of Buffer Solution Effect On Ph The most important of these is undoubtedly the h 2. The buffer capacity considering the effect of dilution, β dil, is defined as the added amount of strong base or strong acid required to change in one unit the ph of an initial v o. Generally, dilution has no effect on the ph. Let us take a look on the. Dilution Of Buffer Solution Effect On Ph.
From www.youtube.com
pH of Buffer Solution (Example) YouTube Dilution Of Buffer Solution Effect On Ph Diluting a buffer solution would decrease its buffer capacity. The most important of these is undoubtedly the h 2. If you look at the buffer formula, ph = pka + lg [salt]/[acid], dilution does. The buffer capacity considering the effect of dilution, β dil, is defined as the added amount of strong base or strong acid required to change in. Dilution Of Buffer Solution Effect On Ph.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Dilution Of Buffer Solution Effect On Ph Generally, dilution has no effect on the ph. The buffer capacity considering the effect of dilution, β dil, is defined as the added amount of strong base or strong acid required to change in one unit the ph of an initial v o. Let us take a look on the following example in the. Diluting a buffer solution would decrease. Dilution Of Buffer Solution Effect On Ph.
From www.brainkart.com
Buffers Dilution Of Buffer Solution Effect On Ph The effects of dilution on the ph of a strong acid (unbuffered solution) and a weak acidic buffer are shown in the diagram below. The most important of these is undoubtedly the h 2. Generally, dilution has no effect on the ph. Diluting a buffer solution would decrease its buffer capacity. If you look at the buffer formula, ph =. Dilution Of Buffer Solution Effect On Ph.